Is Cesium A Transition Metal
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caesium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Culling proper name | cesium (The states) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Advent | pale gilt | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight A r°(Cs) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caesium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diminutive number (Z) | 55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cake | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 6sone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | two, 8, 18, eighteen, 8, one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stage atSTP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 301.7 K (28.v °C, 83.3 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling betoken | 944 K (671 °C, 1240 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (nearr.t.) | one.93 g/cmthree | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (atk.p.) | 1.843 one thousand/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Disquisitional indicate | 1938 K, 9.4 MPa[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oestrus of fusion | 2.09 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 63.nine kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar estrus capacity | 32.210 J/(mol·Chiliad) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapour pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic backdrop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 [3] (a strongly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diminutive radius | empirical: 265 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 244±11 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 343 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other backdrop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centred cubic (bcc) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 97 µm/(one thousand⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 35.nine Due west/(m⋅Yard) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 205 nΩ⋅grand (at twenty °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | ane.vii GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 1.6 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 0.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 0.fourteen MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-46-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | from Latin caesius , sky blue, for its spectral colours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Robert Bunsen and Gustav Kirchhoff (1860) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Offset isolation | Carl Setterberg (1882) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Primary isotopes of caesium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Caesium (IUPAC spelling[six]) (or cesium in American English language)[notation 1] is a element with the symbol Cs and diminutive number 55. Information technology is a soft, argent-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it 1 of only five elemental metals that are liquid at or nigh room temperature.[notation 2] Caesium has physical and chemical backdrop similar to those of rubidium and potassium. The well-nigh reactive of all metals, it is pyrophoric and reacts with h2o fifty-fifty at −116 °C (−177 °F). Information technology is the least electronegative chemical element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The chemical element has 40 known isotopes, making it, along with barium and mercury, 1 of the elements with the nearly isotopes.[11] Caesium-137, a fission product, is extracted from waste produced by nuclear reactors.[ why? ]
The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The get-go minor applications for caesium were every bit a "getter" in vacuum tubes and in photoelectric cells. In 1967, interim on Einstein'south proof that the speed of light is the near constant dimension in the universe, the International System of Units used two specific wave counts from an emission spectrum of caesium-133 to co-define the second and the metre. Since and then, caesium has been widely used in highly authentic diminutive clocks.
Since the 1990s, the largest awarding of the element has been equally caesium formate for drilling fluids, but information technology has a range of applications in the production of electricity, in electronics, and in chemical science. The radioactive isotope caesium-137 has a half-life of about xxx years and is used in medical applications, industrial gauges, and hydrology. Nonradioactive caesium compounds are just mildly toxic, but the pure metal's tendency to react explosively with h2o means that caesium is considered a hazardous fabric, and the radioisotopes present a significant health and ecological take a chance in the environment.
Characteristics [edit]
Physical properties [edit]

Loftier-purity caesium-133 stored in argon.
Of all elements that are solid at room temperature, caesium is the softest: it has a hardness of 0.ii Mohs. It is a very ductile, pale metal, which darkens in the presence of trace amounts of oxygen.[12] [13] [14] When in the presence of mineral oil (where it is all-time kept during ship), it loses its metallic lustre and takes on a duller, grey appearance. It has a melting point of 28.five °C (83.three °F), making information technology i of the few elemental metals that are liquid near room temperature. Mercury is the only stable elemental metallic with a known melting signal lower than caesium.[notation 3] [16] In addition, the metal has a rather low boiling bespeak, 641 °C (one,186 °F), the lowest of all metals other than mercury.[17] Its compounds burn with a blue[eighteen] [19] or violet[19] colour.

Caesium crystals (aureate) compared to rubidium crystals (silver)
Caesium forms alloys with the other alkali metals, gilt, and mercury (amalgams). At temperatures below 650 °C (one,202 °F), information technology does non alloy with cobalt, iron, molybdenum, nickel, platinum, tantalum, or tungsten. It forms well-defined intermetallic compounds with antimony, gallium, indium, and thorium, which are photosensitive.[12] It mixes with all the other brine metals (except lithium); the alloy with a molar distribution of 41% caesium, 47% potassium, and 12% sodium has the lowest melting point of any known metal blend, at −78 °C (−108 °F).[sixteen] [20] A few amalgams have been studied: CsHg
two is black with a purple metal lustre, while CsHg is golden-coloured, likewise with a metallic lustre.[21]
The gilded colour of caesium comes from the decreasing frequency of low-cal required to excite electrons of the alkali metals equally the group is descended. For lithium through rubidium this frequency is in the ultraviolet, but for caesium information technology enters the blue–violet end of the spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium. Thus caesium transmits and partially absorbs violet light preferentially while other colours (having lower frequency) are reflected; hence information technology appears yellowish.[22]
Chemical properties [edit]
Improver of a small amount of caesium to common cold h2o is explosive.
Caesium metal is highly reactive and very pyrophoric. It ignites spontaneously in air, and reacts explosively with water even at depression temperatures, more then than the other brine metals (outset group of the periodic tabular array).[12] Information technology reacts with ice at temperatures as low as −116 °C (−177 °F).[16] Because of this high reactivity, caesium metallic is classified as a hazardous material. Information technology is stored and shipped in dry, saturated hydrocarbons such as mineral oil. It tin be handled only nether inert gas, such as argon. However, a caesium-water explosion is often less powerful than a sodium-water explosion with a like corporeality of sodium. This is because caesium explodes instantly upon contact with water, leaving footling time for hydrogen to accumulate.[23] Caesium can be stored in vacuum-sealed borosilicate glass ampoules. In quantities of more about 100 grams (3.5 oz), caesium is shipped in hermetically sealed, stainless steel containers.[12]
The chemistry of caesium is like to that of other brine metals, in particular rubidium, the element above caesium in the periodic tabular array.[24] Equally expected for an alkali metal, the merely common oxidation country is +ane.[annotation 4] Some slight differences arise from the fact that it has a higher diminutive mass and is more than electropositive than other (nonradioactive) alkali metals.[26] Caesium is the almost electropositive element.[notation 5] [16] The caesium ion is also larger and less "hard" than those of the lighter brine metals.
Compounds [edit]
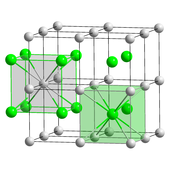
Ball-and-stick model of the cubic coordination of Cs and Cl in CsCl
Most caesium compounds contain the chemical element every bit the cation Cs +
, which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (Cs −
),[three] and others are the several suboxides (encounter section on oxides below). More recently, caesium is predicted to acquit every bit a p-block element and capable of forming higher fluorides with higher oxidation states (i.east., CsFnorthward with due north > 1) under loftier pressure.[28] This prediction needs to exist validated by farther experiments.[29]
Salts of Cs+ are unremarkably colourless unless the anion itself is coloured. Many of the elementary salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate,[30] acetate, carbonate, halides, oxide, nitrate, and sulfate salts are water-soluble. Double salts are often less soluble, and the low solubility of caesium aluminium sulfate is exploited in refining Cs from ores. The double common salt with antimony (such as CsSbCl
4 ), bismuth, cadmium, copper, iron, and pb are also poorly soluble.[12]
Caesium hydroxide (CsOH) is hygroscopic and strongly bones.[24] Information technology apace etches the surface of semiconductors such as silicon.[31] CsOH has been previously regarded past chemists as the "strongest base of operations", reflecting the relatively weak attraction between the large Cs+ ion and OH−;[xviii] it is indeed the strongest Arrhenius base; yet, a number of compounds such every bit n-butyllithium, sodium amide, sodium hydride, caesium hydride, etc., which cannot be dissolved in water every bit reacting violently with information technology simply rather but used in some anhydrous polar aprotic solvents, are far more than basic on the basis of the Brønsted–Lowry acrid–base theory.[24]
A stoichiometric mixture of caesium and gilt will react to form yellow caesium auride (Cs+Au−) upon heating. The auride anion here behaves as a pseudohalogen. The compound reacts violently with water, yielding caesium hydroxide, metallic gold, and hydrogen gas; in liquid ammonia it tin be reacted with a caesium-specific ion substitution resin to produce tetramethylammonium auride. The analogous platinum compound, red caesium platinide (Cs2Pt), contains the platinide ion that behaves as a pseudochalcogen.[32]
Complexes [edit]
Like all metal cations, Cs+ forms complexes with Lewis bases in solution. Considering of its big size, Cs+ unremarkably adopts coordination numbers greater than 6, the number typical for the smaller element of group i cations. This difference is apparent in the 8-coordination of CsCl. This loftier coordination number and softness (trend to form covalent bonds) are properties exploited in separating Cs+ from other cations in the remediation of nuclear wastes, where 137Cs+ must be separated from large amounts of nonradioactive Thou+.[33]
Halides [edit]
Caesium fluoride (CsF) is a hygroscopic white solid that is widely used in organofluorine chemistry as a source of fluoride anions.[35] Caesium fluoride has the halite construction, which ways that the Cs+ and F− pack in a cubic closest packed array every bit do Na+ and Cl− in sodium chloride.[24] Notably, caesium and fluorine have the lowest and highest electronegativities, respectively, amidst all the known elements.
Caesium chloride (CsCl) crystallizes in the simple cubic crystal system. Too chosen the "caesium chloride structure",[26] this structural motif is composed of a primitive cubic lattice with a two-atom footing, each with an eightfold coordination; the chloride atoms prevarication upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the middle of the cubes. This construction is shared with CsBr and CsI, and many other compounds that do not incorporate Cs. In dissimilarity, well-nigh other alkali metal halides have the sodium chloride (NaCl) structure.[26] The CsCl structure is preferred because Cs+ has an ionic radius of 174 pm and Cl −
181 pm.[36]
Oxides [edit]

More so than the other brine metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide CsO
two is the primary product.[37] The "normal" caesium oxide (Cs
2 O) forms yellow-orange hexagonal crystals,[38] and is the only oxide of the anti-CdCl
2 type.[39] Information technology vaporizes at 250 °C (482 °F), and decomposes to caesium metallic and the peroxide Cs
2 O
two at temperatures above 400 °C (752 °F). In improver to the superoxide and the ozonide CsO
three ,[40] [41] several brightly coloured suboxides accept too been studied.[42] These include Cs
7 O, Cs
four O, Cs
eleven O
three , Cs
three O (dark-green[43]), CsO, Cs
three O
2 ,[44] besides as Cs
7 O
2 .[45] [46] The latter may be heated in a vacuum to generate Cs
2 O.[39] Binary compounds with sulfur, selenium, and tellurium likewise be.[12]
Isotopes [edit]
Caesium has 40 known isotopes, ranging in mass number (i.e. number of nucleons in the nucleus) from 112 to 151. Several of these are synthesized from lighter elements past the slow neutron capture procedure (South-process) inside old stars[47] and by the R-process in supernova explosions.[48] The only stable caesium isotope is 133Cs, with 78 neutrons. Although it has a large nuclear spin ( 7 / 2 +), nuclear magnetic resonance studies can use this isotope at a resonating frequency of 11.seven MHz.[49]

The radioactive 135Cs has a very long half-life of well-nigh two.iii million years, the longest of all radioactive isotopes of caesium. 137Cs and 134Cs have half-lives of 30 and two years, respectively. 137Cs decomposes to a brusk-lived 137mBa by beta decay, and then to nonradioactive barium, while 134Cs transforms into 134Ba straight. The isotopes with mass numbers of 129, 131, 132 and 136, have half-lives betwixt a solar day and two weeks, while most of the other isotopes have half-lives from a few seconds to fractions of a second. At least 21 metastable nuclear isomers exist. Other than 134mCs (with a half-life of but under iii hours), all are very unstable and decay with half-lives of a few minutes or less.[50] [51]
The isotope 135Cs is 1 of the long-lived fission products of uranium produced in nuclear reactors.[52] However, this fission product yield is reduced in nigh reactors because the predecessor, 135Xe, is a potent neutron poisonous substance and frequently transmutes to stable 136Xe before it can decay to 135Cs.[53] [54]
The beta decay from 137Cs to 137mBa is a strong emission of gamma radiation.[55] 137Cs and ninetySr are the chief medium-lived products of nuclear fission, and the prime number sources of radioactivity from spent nuclear fuel after several years of cooling, lasting several hundred years.[56] Those two isotopes are the largest source of residual radioactivity in the area of the Chernobyl disaster.[57] Considering of the low capture rate, disposing of 137Cs through neutron capture is not viable and the but electric current solution is to allow it to disuse over time.[58]
Almost all caesium produced from nuclear fission comes from the beta decay of originally more neutron-rich fission products, passing through diverse isotopes of iodine and xenon.[59] Because iodine and xenon are volatile and can diffuse through nuclear fuel or air, radioactive caesium is often created far from the original site of fission.[lx] With nuclear weapons testing in the 1950s through the 1980s, 137Cs was released into the atmosphere and returned to the surface of the world every bit a component of radioactive fallout. It is a ready marker of the movement of soil and sediment from those times.[12]
Occurrence [edit]

Pollucite, a caesium mineral
Caesium is a relatively rare chemical element, estimated to average 3 parts per million in the Globe's crust.[61] It is the 45th most abundant element and the 36th amongst the metals. Nevertheless, it is more abundant than such elements every bit antimony, cadmium, can, and tungsten, and two orders of magnitude more than arable than mercury and silver; it is 3.3% as arable equally rubidium, with which information technology is closely associated, chemically.[12]
Due to its large ionic radius, caesium is i of the "incompatible elements".[62] During magma crystallization, caesium is concentrated in the liquid stage and crystallizes final. Therefore, the largest deposits of caesium are zone pegmatite ore bodies formed by this enrichment process. Because caesium does not substitute for potassium equally readily as rubidium does, the alkali evaporite minerals sylvite (KCl) and carnallite (KMgCl
three ·6H
2 O) may comprise just 0.002% caesium. Consequently, caesium is institute in few minerals. Per centum amounts of caesium may be found in beryl (Exist
three Al
2 (SiO
iii )
6 ) and avogadrite ((G,Cs)BF
four ), upward to 15 wt% Cs2O in the closely related mineral pezzottaite (Cs(Exist
two Li)Al
2 Si
vi O
eighteen ), up to 8.iv wt% Cs2O in the rare mineral londonite ((Cs,K)Al
four Be
iv (B,Be)
12 O
28 ), and less in the more than widespread rhodizite.[12] The only economically important ore for caesium is pollucite Cs(AlSi
two O
six ), which is plant in a few places around the world in zoned pegmatites, associated with the more than commercially important lithium minerals, lepidolite and petalite. Within the pegmatites, the large grain size and the strong separation of the minerals results in high-grade ore for mining.[63]
The earth's near pregnant and richest known source of caesium is the Tanco Mine at Bernic Lake in Manitoba, Canada, estimated to contain 350,000 metric tons of pollucite ore, representing more two-thirds of the globe'southward reserve base.[63] [64] Although the stoichiometric content of caesium in pollucite is 42.six%, pure pollucite samples from this deposit contain just about 34% caesium, while the average content is 24 wt%.[64] Commercial pollucite contains more than 19% caesium.[65] The Bikita pegmatite deposit in Zimbabwe is mined for its petalite, but it also contains a significant amount of pollucite. Another notable source of pollucite is in the Karibib Desert, Namibia.[64] At the nowadays rate of globe mine production of v to ten metric tons per year, reserves volition last for thousands of years.[12]
Production [edit]
Mining and refining pollucite ore is a selective procedure and is conducted on a smaller calibration than for most other metals. The ore is crushed, hand-sorted, but non ordinarily concentrated, and so ground. Caesium is then extracted from pollucite primarily by iii methods: acid digestion, alkaline decomposition, and straight reduction.[12] [66]
In the acid digestion, the silicate pollucite rock is dissolved with strong acids, such every bit hydrochloric (HCl), sulfuric (H
2 SO
4 ), hydrobromic (HBr), or hydrofluoric (HF) acids. With hydrochloric acrid, a mixture of soluble chlorides is produced, and the insoluble chloride double salts of caesium are precipitated equally caesium antimony chloride (Cs
iv SbCl
7 ), caesium iodine chloride (Cs
2 ICl), or caesium hexachlorocerate (Cs
2 (CeCl
6 )). Afterwards separation, the pure precipitated double salt is decomposed, and pure CsCl is precipitated by evaporating the water.
The sulfuric acid method yields the insoluble double salt directly as caesium alum (CsAl(SO
4 )
2 ·12H
two O). The aluminium sulfate component is converted to insoluble aluminium oxide by roasting the alum with carbon, and the resulting product is leached with h2o to yield a Cs
ii SO
4 solution.[12]
Roasting pollucite with calcium carbonate and calcium chloride yields insoluble calcium silicates and soluble caesium chloride. Leaching with water or dilute ammonia (NH
4 OH) yields a dilute chloride (CsCl) solution. This solution tin be evaporated to produce caesium chloride or transformed into caesium alum or caesium carbonate. Though non commercially feasible, the ore can be directly reduced with potassium, sodium, or calcium in vacuum to produce caesium metal straight.[12]
Most of the mined caesium (equally salts) is directly converted into caesium formate (HCOO−Cs+) for applications such as oil drilling. To supply the developing market, Cabot Corporation built a production plant in 1997 at the Tanco mine near Bernic Lake in Manitoba, with a capacity of 12,000 barrels (1,900 m3) per twelvemonth of caesium formate solution.[67] The primary smaller-scale commercial compounds of caesium are caesium chloride and nitrate.[68]
Alternatively, caesium metal may exist obtained from the purified compounds derived from the ore. Caesium chloride and the other caesium halides tin exist reduced at 700 to 800 °C (1,292 to 1,472 °F) with calcium or barium, and caesium metal distilled from the result. In the same way, the aluminate, carbonate, or hydroxide may exist reduced by magnesium.[12]
The metal can likewise be isolated by electrolysis of fused caesium cyanide (CsCN). Exceptionally pure and gas-free caesium tin can be produced by 390 °C (734 °F) thermal decomposition of caesium azide CsN
iii , which can exist produced from aqueous caesium sulfate and barium azide.[66] In vacuum applications, caesium dichromate tin can be reacted with zirconium to produce pure caesium metal without other gaseous products.[68]
- Cs
2 Cr
two O
7 + 2 Zr → two Cs + 2 ZrO
two + Cr
2 O
3
The price of 99.eight% pure caesium (metal footing) in 2009 was well-nigh $10 per gram ($280/oz), but the compounds are significantly cheaper.[64]
History [edit]

In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Dürkheim, Federal republic of germany. Because of the vivid blue lines in the emission spectrum, they derived the name from the Latin word caesius, pregnant sky-blue.[note half dozen] [69] [lxx] [71] Caesium was the first element to be discovered with a spectroscope, which had been invented past Bunsen and Kirchhoff just a year previously.[xvi]
To obtain a pure sample of caesium, 44,000 litres (9,700 imp gal; 12,000 US gal) of mineral water had to be evaporated to yield 240 kilograms (530 lb) of full-bodied salt solution. The alkaline earth metals were precipitated either every bit sulfates or oxalates, leaving the alkali metal in the solution. After conversion to the nitrates and extraction with ethanol, a sodium-free mixture was obtained. From this mixture, the lithium was precipitated past ammonium carbonate. Potassium, rubidium, and caesium grade insoluble salts with chloroplatinic acid, but these salts show a slight difference in solubility in hot water, and the less-soluble caesium and rubidium hexachloroplatinate ((Cs,Rb)2PtCl6) were obtained past fractional crystallization. Later on reduction of the hexachloroplatinate with hydrogen, caesium and rubidium were separated by the difference in solubility of their carbonates in booze. The process yielded nine.2 grams (0.32 oz) of rubidium chloride and vii.3 grams (0.26 oz) of caesium chloride from the initial 44,000 litres of mineral water.[70]
From the caesium chloride, the two scientists estimated the atomic weight of the new chemical element at 123.35 (compared to the currently accustomed i of 132.9).[70] They tried to generate elemental caesium past electrolysis of molten caesium chloride, but instead of a metal, they obtained a blue homogeneous substance which "neither under the naked eye nor under the microscope showed the slightest trace of metal substance"; as a upshot, they assigned information technology equally a subchloride (Cs
ii Cl). In reality, the product was probably a colloidal mixture of the metal and caesium chloride.[72] The electrolysis of the aqueous solution of chloride with a mercury cathode produced a caesium constructing which readily decomposed under the aqueous atmospheric condition.[70] The pure metal was eventually isolated past the German chemist Carl Setterberg while working on his doctorate with Kekulé and Bunsen.[71] In 1882, he produced caesium metal by electrolysing caesium cyanide, fugitive the problems with the chloride.[73]
Historically, the well-nigh of import use for caesium has been in research and development, primarily in chemical and electrical fields. Very few applications existed for caesium until the 1920s, when it came into employ in radio vacuum tubes, where it had 2 functions; equally a getter, information technology removed excess oxygen after manufacture, and as a blanket on the heated cathode, it increased the electrical conductivity. Caesium was not recognized as a high-performance industrial metal until the 1950s.[74] Applications for nonradioactive caesium included photoelectric cells, photomultiplier tubes, optical components of infrared spectrophotometers, catalysts for several organic reactions, crystals for scintillation counters, and in magnetohydrodynamic power generators.[12] Caesium is too used as a source of positive ions in secondary ion mass spectrometry (SIMS).
Since 1967, the International System of Measurements has based the chief unit of measurement of time, the 2nd, on the properties of caesium. The International Arrangement of Units (SI) defines the second as the duration of ix,192,631,770 cycles at the microwave frequency of the spectral line respective to the transition between ii hyperfine energy levels of the ground state of caesium-133.[75] The 13th General Conference on Weights and Measures of 1967 divers a 2d as: "the elapsing of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground land undisturbed by external fields".
Applications [edit]
Petroleum exploration [edit]
The largest present-mean solar day apply of nonradioactive caesium is in caesium formate drilling fluids for the extractive oil industry.[12] Aqueous solutions of caesium formate (HCOO−Cs+)—made by reacting caesium hydroxide with formic acid—were developed in the mid-1990s for utilize as oil well drilling and completion fluids. The function of a drilling fluid is to lubricate drill bits, to bring stone cuttings to the surface, and to maintain pressure on the formation during drilling of the well. Completion fluids assist the emplacement of control hardware after drilling only prior to production by maintaining the pressure level.[12]
The loftier density of the caesium formate brine (upwardly to 2.3 g/cm3, or nineteen.2 pounds per gallon),[76] coupled with the relatively benign nature of about caesium compounds, reduces the requirement for toxic high-density suspended solids in the drilling fluid—a significant technological, applied science and environmental advantage. Dissimilar the components of many other heavy liquids, caesium formate is relatively environment-friendly.[76] Caesium formate alkali can exist blended with potassium and sodium formates to decrease the density of the fluids to that of water (one.0 thou/cm3, or 8.3 pounds per gallon). Furthermore, it is biodegradable and may exist recycled, which is important in view of its loftier price (almost $four,000 per butt in 2001).[77] Alkali formates are safe to handle and practice not damage the producing formation or downhole metals as corrosive alternative, high-density brines (such as zinc bromide ZnBr
2 solutions) sometimes do; they too require less cleanup and reduce disposal costs.[12]
Atomic clocks [edit]

Atomic clock ensemble at the U.S. Naval Observatory

FOCS-1, a continuous cold caesium fountain atomic clock in Switzerland, started operating in 2004 at an uncertainty of one second in 30 million years
Caesium-based atomic clocks use the electromagnetic transitions in the hyperfine structure of caesium-133 atoms as a reference bespeak. The first accurate caesium clock was built by Louis Essen in 1955 at the National Physical Laboratory in the United kingdom of great britain and northern ireland.[78] Caesium clocks accept improved over the past one-half-century and are regarded as "the near accurate realization of a unit of measurement that flesh has yet achieved."[75] These clocks measure frequency with an fault of 2 to 3 parts in 1014, which corresponds to an accuracy of 2 nanoseconds per day, or one 2nd in 1.four million years. The latest versions are more than accurate than 1 part in ten15, about 1 second in xx one thousand thousand years.[12] The caesium standard is the primary standard for standards-compliant time and frequency measurements.[79] Caesium clocks regulate the timing of cell phone networks and the Net.[80]
Definition of the 2nd [edit]
The second, symbol s, is the SI unit of time. It is defined past taking the fixed numerical value of the caesium frequency Δν Cs , the unperturbed ground-country hyperfine transition frequency of the caesium-133 atom, to be ix192 631 770 when expressed in the unit Hz, which is equal to south−1.
Electric ability and electronics [edit]
Caesium vapour thermionic generators are depression-power devices that convert heat energy to electrical energy. In the two-electrode vacuum tube converter, caesium neutralizes the infinite charge nigh the cathode and enhances the current menses.[81]
Caesium is also important for its photoemissive properties, converting light to electron flow. It is used in photoelectric cells because caesium-based cathodes, such as the intermetallic compound K
two CsSb, have a low threshold voltage for emission of electrons.[82] The range of photoemissive devices using caesium include optical character recognition devices, photomultiplier tubes, and video camera tubes.[83] [84] Nevertheless, germanium, rubidium, selenium, silicon, tellurium, and several other elements tin be substituted for caesium in photosensitive materials.[12]
Caesium iodide (CsI), bromide (CsBr) and caesium fluoride (CsF) crystals are employed for scintillators in scintillation counters widely used in mineral exploration and particle physics research to observe gamma and X-ray radiation. Being a heavy element, caesium provides practiced stopping power with amend detection. Caesium compounds may provide a faster response (CsF) and exist less hygroscopic (CsI).
Caesium vapour is used in many common magnetometers.[85]
The element is used as an internal standard in spectrophotometry.[86] Like other alkali metals, caesium has a swell affinity for oxygen and is used as a "getter" in vacuum tubes.[87] Other uses of the metallic include high-free energy lasers, vapour glow lamps, and vapour rectifiers.[12]
Centrifugation fluids [edit]
The high density of the caesium ion makes solutions of caesium chloride, caesium sulfate, and caesium trifluoroacetate (Cs(O
ii CCF
three )) useful in molecular biological science for density slope ultracentrifugation.[88] This engineering is used primarily in the isolation of viral particles, subcellular organelles and fractions, and nucleic acids from biological samples.[89]
Chemical and medical use [edit]

Relatively few chemic applications use caesium.[ninety] Doping with caesium compounds enhances the effectiveness of several metallic-ion catalysts for chemical synthesis, such equally acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various olefins. Information technology is too used in the catalytic conversion of sulfur dioxide into sulfur trioxide in the production of sulfuric acid.[12]
Caesium fluoride enjoys a niche employ in organic chemistry as a base[24] and equally an anhydrous source of fluoride ion.[91] Caesium salts sometimes supercede potassium or sodium salts in organic synthesis, such every bit cyclization, esterification, and polymerization. Caesium has also been used in thermoluminescent radiation dosimetry (TLD): When exposed to radiation, information technology acquires crystal defects that, when heated, revert with emission of light proportionate to the received dose. Thus, measuring the lite pulse with a photomultiplier tube tin permit the accumulated radiations dose to be quantified.
Nuclear and isotope applications [edit]
Caesium-137 is a radioisotope unremarkably used equally a gamma-emitter in industrial applications. Its advantages include a one-half-life of roughly thirty years, its availability from the nuclear fuel cycle, and having 137Ba as a stable end product. The high water solubility is a disadvantage which makes it incompatible with large pool irradiators for food and medical supplies.[92] Information technology has been used in agronomics, cancer treatment, and the sterilization of food, sewage sludge, and surgical equipment.[12] [93] Radioactive isotopes of caesium in radiation devices were used in the medical field to treat certain types of cancer,[94] simply emergence of better alternatives and the utilize of water-soluble caesium chloride in the sources, which could create broad-ranging contamination, gradually put some of these caesium sources out of use.[95] [96] Caesium-137 has been employed in a diverseness of industrial measurement gauges, including moisture, density, levelling, and thickness gauges.[97] Information technology has besides been used in well logging devices for measuring the electron density of the rock formations, which is analogous to the bulk density of the formations.[98]
Caesium-137 has been used in hydrologic studies analogous to those with tritium. Every bit a daughter product of fission bomb testing from the 1950s through the mid-1980s, caesium-137 was released into the atmosphere, where it was absorbed readily into solution. Known yr-to-twelvemonth variation within that menstruation allows correlation with soil and sediment layers. Caesium-134, and to a bottom extent caesium-135, have also been used in hydrology to measure out the caesium output by the nuclear power industry. While they are less prevalent than either caesium-133 or caesium-137, these bellwether isotopes are produced solely from anthropogenic sources.[99]
Other uses [edit]

Schematics of an electrostatic ion thruster developed for utilize with caesium or mercury fuel
Caesium and mercury were used as a propellant in early ion engines designed for spacecraft propulsion on very long interplanetary or extraplanetary missions. The fuel was ionized by contact with a charged tungsten electrode. Simply corrosion by caesium on spacecraft components has pushed evolution in the direction of inert gas propellants, such as xenon, which are easier to handle in basis-based tests and do less potential damage to the spacecraft.[12] Xenon was used in the experimental spacecraft Deep Space ane launched in 1998.[100] [101] Nevertheless, field-emission electric propulsion thrusters that advance liquid metal ions such as caesium have been congenital.[102]
Caesium nitrate is used as an oxidizer and pyrotechnic colorant to burn silicon in infrared flares,[103] such every bit the LUU-19 flare,[104] because it emits much of its lite in the nigh infrared spectrum.[105] Caesium compounds may have been used as fuel additives to reduce the radar signature of frazzle plumes in the Lockheed A-12 CIA reconnaissance aircraft.[106] Caesium and rubidium have been added as a carbonate to glass considering they reduce electrical conductivity and improve stability and durability of fibre optics and night vision devices. Caesium fluoride or caesium aluminium fluoride are used in fluxes formulated for brazing aluminium alloys that incorporate magnesium.[12]
Magnetohydrodynamic (MHD) power-generating systems were researched, only failed to gain widespread acceptance.[107] Caesium metal has also been considered as the working fluid in high-temperature Rankine wheel turboelectric generators.[108]
Caesium salts have been evaluated every bit antishock reagents following the assistants of arsenical drugs. Because of their result on eye rhythms, however, they are less probable to be used than potassium or rubidium salts. They have also been used to treat epilepsy.[12]
Caesium-133 can be light amplification by stimulated emission of radiation cooled and used to probe key and technological bug in quantum physics. It has a particularly convenient Feshbach spectrum to enable studies of ultracold atoms requiring tunable interactions.[109]
Health and safety hazards [edit]
| Hazards | |
|---|---|
| GHS labelling:[110] | |
| Pictograms |   |
| Bespeak word | Danger |
| Gamble statements | H260, H314 |
| Precautionary statements | P223, P231+P232, P280, P305+P351+P338, P370+P378, P422 |
| NFPA 704 (burn diamond) | iii 4 3 |

The portion of the total radiation dose (in air) contributed by each isotope plotted against time afterward the Chernobyl disaster. Caesium-137 became the chief source of radiation nearly 200 days after the blow.[111]
Nonradioactive caesium compounds are only mildly toxic, and nonradioactive caesium is not a significant ecology hazard. Considering biochemical processes tin can confuse and substitute caesium with potassium, excess caesium can lead to hypokalemia, arrhythmia, and acute cardiac arrest, simply such amounts would not ordinarily exist encountered in natural sources.[112] [113]
The median lethal dose (LDfifty) for caesium chloride in mice is 2.3 thousand per kilogram, which is comparable to the LD50 values of potassium chloride and sodium chloride.[114] The primary utilise of nonradioactive caesium is every bit caesium formate in petroleum drilling fluids because information technology is much less toxic than alternatives, though it is more costly.[76]
Caesium metallic is i of the virtually reactive elements and is highly explosive in the presence of water. The hydrogen gas produced by the reaction is heated by the thermal energy released at the same time, causing ignition and a violent explosion. This can occur with other alkali metals, but caesium is so potent that this explosive reaction can be triggered even by common cold h2o.[12]
It is highly pyrophoric: the autoignition temperature of caesium is −116 °C (−177 °F), and information technology ignites explosively in air to form caesium hydroxide and various oxides. Caesium hydroxide is a very strong base of operations, and will quickly corrode glass.[17]
The isotopes 134 and 137 are present in the biosphere in pocket-size amounts from human activities, differing past location. Radiocaesium does not accumulate in the torso as readily as other fission products (such equally radioiodine and radiostrontium). About 10% of absorbed radiocaesium washes out of the torso relatively quickly in sweat and urine. The remaining xc% has a biological half-life between fifty and 150 days.[115] Radiocaesium follows potassium and tends to accumulate in plant tissues, including fruits and vegetables.[116] [117] [118] Plants vary widely in the assimilation of caesium, sometimes displaying great resistance to information technology. It is also well-documented that mushrooms from contaminated forests accumulate radiocaesium (caesium-137) in the fungal sporocarps.[119] Aggregating of caesium-137 in lakes has been a great concern later on the Chernobyl disaster.[120] [121] Experiments with dogs showed that a single dose of 3.8 millicuries (140 MBq, 4.i μg of caesium-137) per kilogram is lethal inside three weeks;[122] smaller amounts may crusade infertility and cancer.[123] The International Diminutive Free energy Agency and other sources accept warned that radioactive materials, such as caesium-137, could be used in radiological dispersion devices, or "dirty bombs".[124]
See also [edit]
- Goiânia accident, a major radioactive contamination incident in 1987 involving Caesium-137.
- Kramatorsk radiological accident, another 137Cs incident between 1980 and 1989.
- Acerinox accident, a Caesium-137 contamination accident in 1998.
Notes [edit]
- ^ Caesium is the spelling recommended past the International Wedlock of Pure and Practical Chemistry (IUPAC).[7] The American Chemical Guild (ACS) has used the spelling cesium since 1921,[eight] [9] following Webster'south New International Dictionary. The element was named after the Latin word caesius, significant "blue grey".[x] In medieval and early on modern writings caesius was spelled with the ligature æ equally cæsius; hence, an alternative just now former-fashioned orthography is cæsium. More than spelling explanation at ae/oe vs e.
- ^ Along with rubidium (39 °C [102 °F]), francium (estimated at 27 °C [81 °F]), mercury (−39 °C [−38 °F]), and gallium (30 °C [86 °F]); bromine is also liquid at room temperature (melting at −7.2 °C [19.0 °F]), only information technology is a halogen and non a metal. Preliminary work with copernicium and flerovium suggests that they are gaseous metals at room temperature.
- ^ The radioactive element francium may also have a lower melting point, but its radioactive decay prevents enough of it from being isolated for direct testing.[xv] Copernicium and flerovium may besides have lower melting points.
- ^ It differs from this value in caesides, which contain the Cs− anion and thus have caesium in the −1 oxidation state.[3] Additionally, 2013 calculations by Mao-sheng Miao indicate that under atmospheric condition of extreme pressure (greater than 30 GPa), the inner 5p electrons could form chemic bonds, where caesium would behave as the seventh 5p chemical element. This discovery indicates that college caesium fluorides with caesium in oxidation states from +ii to +6 could exist under such conditions.[25]
- ^ Francium's electropositivity has non been experimentally measured due to its high radioactivity. Measurements of the outset ionization energy of francium advise that its relativistic effects may lower its reactivity and enhance its electronegativity above that expected from periodic trends.[27]
- ^ Bunsen quotes Aulus Gellius Noctes Atticae Ii, 26 by Nigidius Figulus: Nostris autem veteribus caesia dicts est quae Graecis, ut Nigidus ait, de colore coeli quasi coelia.
References [edit]
- ^ "Standard Atomic Weights: Caesium". CIAAW. 2013.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Printing. p. four.121. ISBN1-4398-5511-0.
- ^ a b c Dye, J. L. (1979). "Compounds of Alkali metal Anions". Angewandte Chemie International Edition. eighteen (8): 587–598. doi:10.1002/anie.197905871.
- ^ "Magnetic susceptibility of the elements and inorganic compounds". Handbook of Chemistry and Physics (PDF) (87th ed.). CRC press. ISBN0-8493-0487-3 . Retrieved 2010-09-26 .
- ^ "NIST Radionuclide Half-Life Measurements". NIST . Retrieved 2011-03-thirteen .
- ^ "IUPAC Periodic Table of Elements". International Spousal relationship of Pure and Practical Chemical science.
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemical science (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 248–49. Electronic version..
- ^ Coghill, Anne Thou.; Garson, Lorrin R., eds. (2006). The ACS Mode Guide: Effective Communication of Scientific Information (3rd ed.). Washington, D.C.: American Chemic Society. p. 127. ISBN978-0-8412-3999-9.
- ^ Coplen, T. B.; Peiser, H. Due south. (1998). "History of the recommended atomic-weight values from 1882 to 1997: a comparison of differences from current values to the estimated uncertainties of earlier values" (PDF). Pure Appl. Chem. 70 (1): 237–257. doi:x.1351/pac199870010237. S2CID 96729044.
- ^ OED entry for "caesium". Second edition, 1989; online version June 2012. Retrieved 07 September 2012. Earlier version starting time published in New English Dictionary, 1888.
- ^ "Isotopes". Ptable.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa Butterman, William C.; Brooks, William E.; Reese, Robert Thousand. Jr. (2004). "Mineral Commodity Profile: Cesium" (PDF). United states Geological Survey. Archived from the original (PDF) on February seven, 2007. Retrieved 2009-12-27 .
- ^ Heiserman, David L. (1992). Exploring Chemical Elements and their Compounds . McGraw-Hill. pp. 201–203. ISBN978-0-8306-3015-8.
- ^ Addison, C. C. (1984). The Chemistry of the Liquid Alkali Metals. Wiley. ISBN978-0-471-90508-0 . Retrieved 2012-09-28 .
- ^ "Francium". Periodic.lanl.gov. Retrieved 2010-02-23 .
- ^ a b c d eastward Kaner, Richard (2003). "C&EN: It's Elemental: The Periodic Tabular array – Cesium". American Chemical Social club. Retrieved 2010-02-25 .
- ^ a b "Chemical Data – Caesium – Cs". Royal Society of Chemistry. Retrieved 2010-09-27 .
- ^ a b Lynch, Charles T. (1974). CRC Handbook of Materials Science. CRC Press. p. 13. ISBN978-0-8493-2321-8.
- ^ a b Clark, Jim (2005). "Flame Tests". chemguide . Retrieved 2012-01-29 .
- ^ Taova, T. M.; et al. (June 22, 2003). "Density of melts of alkali metals and their Na-K-Cs and Na-K-Rb ternary systems" (PDF). Fifteenth symposium on thermophysical properties, Boulder, Colorado, United States. Archived from the original (PDF) on October ix, 2006. Retrieved 2010-09-26 .
- ^ Deiseroth, H. J. (1997). "Alkaline amalgams, a group of unusual alloys". Progress in Solid State Chemistry. 25 (1–ii): 73–123. doi:x.1016/S0079-6786(97)81004-7.
- ^ Addison, C. C. (1984). The chemical science of the liquid alkali metals. Wiley. p. 7. ISBN9780471905080.
- ^ Grey, Theodore (2012) The Elements, Black Dog & Leventhal Publishers, p. 131, ISBN 1-57912-895-5.
- ^ a b c d e Greenwood, North. N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford, Uk: Pergamon Printing. ISBN978-0-08-022057-four.
- ^ Moskowitz, Clara. "A Basic Dominion of Chemical science Can Exist Broken, Calculations Prove". Scientific American . Retrieved 2013-eleven-22 .
- ^ a b c Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Vergleichende Übersicht über die Gruppe der Alkalimetalle". Lehrbuch der Anorganischen Chemie (in German language) (91–100 ed.). Walter de Gruyter. pp. 953–955. ISBN978-three-11-007511-3.
- ^ Andreev, Due south. V.; Letokhov, V. S.; Mishin, 5. I. (1987). "Laser resonance photoionization spectroscopy of Rydberg levels in Fr". Concrete Review Letters. 59 (12): 1274–76. Bibcode:1987PhRvL..59.1274A. doi:10.1103/PhysRevLett.59.1274. PMID 10035190.
- ^ Miao, Mao-sheng (2013). "Caesium in high oxidation states and every bit a p-block element". Nature Chemistry. v (10): 846–852. arXiv:1212.6290. Bibcode:2013NatCh...five..846M. doi:10.1038/nchem.1754. ISSN 1755-4349. PMID 24056341. S2CID 38839337.
- ^ Sneed, D.; Pravica, 1000.; Kim, East.; Chen, N.; Park, C.; White, Grand. (2017-10-01). "Forcing Cesium into College Oxidation States Using Useful hard ten-ray Induced Chemistry under High Pressure". Journal of Physics: Briefing Series. 950 (xi, 2017): 042055. Bibcode:2017JPhCS.950d2055S. doi:ten.1088/1742-6596/950/4/042055. ISSN 1742-6588. OSTI 1409108. S2CID 102912809.
- ^ Hogan, C. Yard. (2011)."Phosphate". Archived from the original on 2012-10-25. Retrieved 2012-06-17 . in Encyclopedia of Globe. Jorgensen, A. and Cleveland, C.J. (eds.). National Council for Science and the Environs. Washington DC
- ^ Köhler, Michael J. (1999). Etching in microsystem technology. Wiley-VCH. p. 90. ISBN978-3-527-29561-6.
- ^ Jansen, Martin (2005-11-30). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences. 7 (12): 1464–1474. Bibcode:2005SSSci...7.1464J. doi:10.1016/j.solidstatesciences.2005.06.015.
- ^ Moyer, Bruce A.; Birdwell, Joseph F.; Bonnesen, Peter V.; Delmau, Laetitia H. (2005). Utilize of Macrocycles in Nuclear-Waste Cleanup: A Realworld Application of a Calixcrown in Cesium Separation Technology. Macrocyclic Chemistry. pp. 383–405. doi:10.1007/1-4020-3687-6_24. ISBN978-1-4020-3364-three. .
- ^ Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron free energy loss spectroscopy of calorie-free elements". Nature Communications. half dozen: 7943. Bibcode:2015NatCo...6.7943S. doi:10.1038/ncomms8943. PMC4532884. PMID 26228378.
- ^ Evans, F. W.; Litt, One thousand. H.; Weidler-Kubanek, A. Yard.; Avonda, F. P. (1968). "Reactions Catalyzed by Potassium Fluoride. 111. The Knoevenagel Reaction". Journal of Organic Chemical science. 33 (5): 1837–1839. doi:10.1021/jo01269a028.
- ^ Wells, A. F. (1984). Structural Inorganic Chemical science (5th ed.). Oxford Science Publications. ISBN978-0-19-855370-0.
- ^ Cotton, F. Albert; Wilkinson, 1000. (1962). Avant-garde Inorganic Chemistry. John Wiley & Sons, Inc. p. 318. ISBN978-0-471-84997-1.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 451, 514. ISBN0-8493-0487-iii.
- ^ a b Tsai, Khi-Ruey; Harris, P. M.; Lassettre, E. N. (1956). "The Crystal Structure of Cesium Monoxide". Journal of Physical Chemistry. 60 (iii): 338–344. doi:10.1021/j150537a022. Archived from the original on September 24, 2017.
- ^ Vol'nov, I. I.; Matveev, V. V. (1963). "Synthesis of cesium ozonide through cesium superoxide". Bulletin of the University of Sciences, USSR Division of Chemistry. 12 (6): 1040–1043. doi:x.1007/BF00845494.
- ^ Tokareva, S. A. (1971). "Alkali and Element of group ii Ozonides". Russian Chemical Reviews. twoscore (2): 165–174. Bibcode:1971RuCRv..40..165T. doi:x.1070/RC1971v040n02ABEH001903. S2CID 250883291.
- ^ Simon, A. (1997). "Group ane and 2 Suboxides and Subnitrides — Metals with Atomic Size Holes and Tunnels". Coordination Chemical science Reviews. 163: 253–270. doi:10.1016/S0010-8545(97)00013-1.
- ^ Tsai, Khi-Ruey; Harris, P. M.; Lassettre, E. N. (1956). "The Crystal Structure of Tricesium Monoxide". Journal of Concrete Chemistry. 60 (3): 345–347. doi:10.1021/j150537a023.
- ^ Okamoto, H. (2009). "Cs-O (Cesium-Oxygen)". Journal of Phase Equilibria and Diffusion. 31: 86–87. doi:10.1007/s11669-009-9636-5. S2CID 96084147.
- ^ Band, A.; Albu-Yaron, A.; Livneh, T.; Cohen, H.; Feldman, Y.; Shimon, L.; Popovitz-Biro, R.; Lyahovitskaya, Five.; Tenne, R. (2004). "Characterization of Oxides of Cesium". The Journal of Physical Chemistry B. 108 (33): 12360–12367. doi:10.1021/jp036432o.
- ^ Brauer, Grand. (1947). "Untersuchungen ber das System Csium-Sauerstoff". Zeitschrift für Anorganische Chemie. 255 (1–3): 101–124. doi:10.1002/zaac.19472550110.
- ^ Busso, Grand.; Gallino, R.; Wasserburg, G. J. (1999). "Nucleosynthesis in Asymptotic Behemothic Co-operative Stars: Relevance for Galactic Enrichment and Solar System Formation" (PDF). Annual Review of Astronomy and Astrophysics. 37: 239–309. Bibcode:1999ARA&A..37..239B. doi:ten.1146/annurev.astro.37.one.239. Archived (PDF) from the original on 2022-10-x. Retrieved 2010-02-twenty .
- ^ Arnett, David (1996). Supernovae and Nucleosynthesis: An Investigation of the History of Affair, from the Large Bang to the Nowadays. Princeton Academy Press. p. 527. ISBN978-0-691-01147-9.
- ^ Goff, C.; Matchette, Michael A.; Shabestary, Nahid; Khazaeli, Sadegh (1996). "Complexation of caesium and rubidium cations with crown ethers in N,Due north-dimethylformamide". Polyhedron. 15 (21): 3897–3903. doi:10.1016/0277-5387(96)00018-half-dozen.
- ^ Brown, F.; Hall, G. R.; Walter, A. J. (1955). "The one-half-life of Cs137". Journal of Inorganic and Nuclear Chemical science. 1 (iv–5): 241–247. Bibcode:1955PhRv...99..188W. doi:10.1016/0022-1902(55)80027-9.
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Archived from the original on 2008-05-22. Retrieved 2008-06-06 .
- ^ Ohki, Shigeo; Takaki, Naoyuki (14–sixteen October 2002). Transmutation of Cesium-135 with Fast Reactors (PDF). 7th Data Exchange Meeting on Actinide and Fission Product Sectionalisation and Transmutation. Jeju, Korea. Archived from the original (PDF) on 2011-09-28. Retrieved 2010-09-26 .
- ^ "20 Xenon: A Fission Production Poison" (PDF). CANDU Fundamentals (Study). CANDU Owners Grouping Inc. Archived from the original (PDF) on July 23, 2011. Retrieved 2010-09-xv .
- ^ Taylor, V. F.; Evans, R. D.; Cornett, R. J. (2008). "Preliminary evaluation of 135Cs/137Cs every bit a forensic tool for identifying source of radioactive contamination". Periodical of Ecology Radioactivity. 99 (one): 109–118. doi:ten.1016/j.jenvrad.2007.07.006. PMID 17869392.
- ^ "Cesium | Radiation Protection". U.S. Environmental Protection Bureau. 2006-06-28. Archived from the original on March 15, 2011. Retrieved 2010-02-15 .
- ^ Zerriffi, Hisham (2000-05-24). IEER Report: Transmutation – Nuclear Alchemy Adventure (Report). Institute for Energy and Environmental Inquiry. Retrieved 2010-02-15 .
- ^ Chernobyl'south Legacy: Health, Environmental and Socia-Economic Impacts and Recommendations to the Governments of Republic of belarus, Russia and Ukraine (PDF) (Written report). International Diminutive Free energy Agency. Archived from the original (PDF) on 2010-02-xv. Retrieved 2010-02-xviii .
- ^ Kase, Takeshi; Konashi, Kenji; Takahashi, Hiroshi; Hirao, Yasuo (1993). "Transmutation of Cesium-137 Using Proton Accelerator". Periodical of Nuclear Science and Technology. 30 (9): 911–918. doi:ten.3327/jnst.30.911.
- ^ Knief, Ronald Allen (1992). "Fission Fragments". Nuclear engineering: theory and technology of commercial nuclear power. Taylor & Francis. p. 42. ISBN978-1-56032-088-3.
- ^ Ishiwatari, N.; Nagai, H. "Release of xenon-137 and iodine-137 from UO2 pellet by pulse neutron irradiation at NSRR". Nippon Genshiryoku Gakkaishi. 23 (11): 843–850. OSTI 5714707.
- ^ Turekian, Grand. K.; Wedepohl, K. H. (1961). "Distribution of the elements in some major units of the Earth's crust". Geological Society of America Bulletin. 72 (two): 175–192. Bibcode:1961GSAB...72..175T. doi:10.1130/0016-7606(1961)72[175:DOTEIS]ii.0.CO;two. ISSN 0016-7606.
- ^ Rowland, Simon (1998-07-04). "Cesium equally a Raw Material: Occurrence and Uses". Artemis Society International. Retrieved 2010-02-xv .
- ^ a b Černý, Petr; Simpson, F. M. (1978). "The Tanco Pegmatite at Bernic Lake, Manitoba: X. Pollucite" (PDF). Canadian Mineralogist. 16: 325–333. Archived (PDF) from the original on 2022-10-10. Retrieved 2010-09-26 .
- ^ a b c d Polyak, Désirée Eastward. "Cesium" (PDF). U.South. Geological Survey. Archived (PDF) from the original on 2009-05-08. Retrieved 2009-x-17 .
- ^ Norton, J. J. (1973). "Lithium, cesium, and rubidium—The rare alkali metals". In Brobst, D. A.; Pratt, W. P. (eds.). U.s.a. mineral resources. Vol. Newspaper 820. U.S. Geological Survey Professional. pp. 365–378. Archived from the original on 2010-07-21. Retrieved 2010-09-26 .
- ^ a b Burt, R. O. (1993). "Caesium and cesium compounds". Kirk-Othmer encyclopedia of chemical engineering. Vol. 5 (4th ed.). New York: John Wiley & Sons, Inc. pp. 749–764. ISBN978-0-471-48494-iii.
- ^ Benton, William; Turner, Jim (2000). "Cesium formate fluid succeeds in North Bounding main HPHT field trials" (PDF). Drilling Contractor (May/June): 38–41. Retrieved 2010-09-26 .
- ^ a b Eagleson, Mary, ed. (1994). Concise encyclopedia chemistry. Eagleson, Mary. Berlin: de Gruyter. p. 198. ISBN978-3-xi-011451-five.
- ^ Oxford English language Dictionary, second Edition
- ^ a b c d Kirchhoff, G.; Bunsen, R. (1861). "Chemische Analyse durch Spectralbeobachtungen" (PDF). Annalen der Physik und Chemie. 189 (7): 337–381. Bibcode:1861AnP...189..337K. doi:10.1002/andp.18611890702. hdl:2027/hvd.32044080591324.
- ^ a b Weeks, Mary Elvira (1932). "The discovery of the elements. XIII. Some spectroscopic discoveries". Journal of Chemical Didactics. 9 (viii): 1413–1434. Bibcode:1932JChEd...9.1413W. doi:10.1021/ed009p1413.
- ^ Zsigmondy, Richard (2007). Colloids and the Ultra Microscope. Read books. p. 69. ISBN978-1-4067-5938-9.
- ^ Setterberg, Carl (1882). "Ueber die Darstellung von Rubidium- und Cäsiumverbindungen und über die Gewinnung der Metalle selbst". Justus Liebig'southward Annalen der Chemie. 211: 100–116. doi:10.1002/jlac.18822110105.
- ^ Strod, A. J. (1957). "Cesium—A new industrial metal". American Ceramic Bulletin. 36 (vi): 212–213.
- ^ a b "Cesium Atoms at Work". Time Service Department—U.S. Naval Observatory—Department of the Navy. Archived from the original on February 23, 2015. Retrieved 2009-12-xx .
- ^ a b c Downs, J. D.; Blaszczynski, M.; Turner, J.; Harris, M. (February 2006). Drilling and Completing Difficult HP/HT Wells With the Aid of Cesium Formate Brines-A Performance Review. IADC/SPE Drilling Conference. Miami, Florida, USASociety of Petroleum Engineers. doi:10.2118/99068-MS. Archived from the original on 2007-10-12.
- ^ Flatern, Rick (2001). "Keeping cool in the HPHT environment". Offshore Engineer (February): 33–37.
- ^ Essen, L.; Parry, J. V. 50. (1955). "An Atomic Standard of Frequency and Time Interval: A Caesium Resonator". Nature. 176 (4476): 280–282. Bibcode:1955Natur.176..280E. doi:10.1038/176280a0. S2CID 4191481.
- ^ Markowitz, Westward.; Hall, R.; Essen, L.; Parry, J. (1958). "Frequency of Cesium in Terms of Ephemeris Time". Physical Review Letters. 1 (3): 105–107. Bibcode:1958PhRvL...1..105M. doi:10.1103/PhysRevLett.1.105.
- ^ Reel, Monte (2003-07-22). "Where timing truly is everything". The Washington Post. p. B1. Archived from the original on 2013-04-29. Retrieved 2010-01-26 .
- ^ Rasor, Ned S.; Warner, Charles (September 1964). "Correlation of Emission Processes for Adsorbed Alkali Films on Metallic Surfaces". Periodical of Applied Physics. 35 (9): 2589–2600. Bibcode:1964JAP....35.2589R. doi:x.1063/1.1713806.
- ^ "Cesium Supplier & Technical Information". American Elements. Retrieved 2010-01-25 .
- ^ Smedley, John; Rao, Triveni; Wang, Erdong (2009). "G2CsSb Cathode Development". AIP Conference Proceedings. 1149 (one): 1062–1066. Bibcode:2009AIPC.1149.1062S. doi:10.1063/1.3215593.
- ^ Görlich, P. (1936). "Über zusammengesetzte, durchsichtige Photokathoden". Zeitschrift für Physik. 101 (v–6): 335–342. Bibcode:1936ZPhy..101..335G. doi:10.1007/BF01342330. S2CID 121613539.
- ^ Groeger, South.; Pazgalev, A. S.; Weis, A. (2005). "Comparison of discharge lamp and laser pumped cesium magnetometers". Applied Physics B. lxxx (6): 645–654. arXiv:physics/0412011. Bibcode:2005ApPhB..80..645G. doi:x.1007/s00340-005-1773-x. S2CID 36065775.
- ^ Haven, Mary C.; Tetrault, Gregory A.; Schenken, Jerald R. (1994). "Internal Standards". Laboratory instrumentation. New York: John Wiley and Sons. p. 108. ISBN978-0-471-28572-4.
- ^ McGee, James D. (1969). Photo-electronic epitome devices: proceedings of the quaternary symposium held at Imperial Higher, London, September sixteen–20, 1968. Vol. 1. Academic Press. p. 391. ISBN978-0-12-014528-seven.
- ^ Manfred Bick, Horst Prinz, "Cesium and Cesium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_153.
- ^ Desai, Mohamed A., ed. (2000). "Gradient Materials". Downstream processing methods. Totowa, N.J.: Humana Printing. pp. 61–62. ISBN978-0-89603-564-5.
- ^ Burt, R. O. (1993). "Cesium and cesium compounds". Kirk-Othmer encyclopedia of chemic technology. Vol. v (4th ed.). New York: John Wiley & Sons. p. 759. ISBN978-0-471-15158-six.
- ^ Friestad, Gregory Grand.; Branchaud, Bruce P.; Navarrini, Walter and Sansotera, Maurizio (2007) "Cesium Fluoride" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons. doi:10.1002/047084289X.rc050.pub2
- ^ Okumura, Takeshi (2003-ten-21). "The textile flow of radioactive cesium-137 in the U.S. 2000" (PDF). Us Environmental Protection Agency. Archived from the original (PDF) on July 20, 2011. Retrieved 2009-12-20 .
- ^ Jensen, N. L. (1985). "Cesium". Mineral facts and problems. Vol. Bulletin 675. U.S. Bureau of Mines. pp. 133–138.
- ^ "IsoRay's Cesium-131 Medical Isotope Used In Milestone Process Treating Eye Cancers At Tufts-New England Medical Center". Medical News Today. 2007-12-17. Retrieved 2010-02-15 .
- ^ Bentel, Gunilla Carleson (1996). "Caesium-137 Machines". Radiation therapy planning. McGraw-Hill Professional. pp. 22–23. ISBN978-0-07-005115-vii . Retrieved 2010-09-26 .
- ^ National Inquiry Council (U.S.). Committee on Radiations Source Apply and Replacement (2008). Radiation source utilize and replacement: abbreviated version. National Academies Press. ISBN978-0-309-11014-iii.
- ^ Loxton, R.; Pope, P., eds. (1995). "Level and density measurement using non-contact nuclear gauges". Instrumentation : A Reader. London: Chapman & Hall. pp. 82–85. ISBN978-0-412-53400-3.
- ^ Timur, A.; Toksoz, M. N. (1985). "Downhole Geophysical Logging". Annual Review of Earth and Planetary Sciences. 13: 315–344. Bibcode:1985AREPS..xiii..315T. doi:10.1146/annurev.ea.13.050185.001531.
- ^ Kendall, Ballad. "Isotope Tracers Project – Resource on Isotopes – Cesium". National Research Program – U.Southward. Geological Survey. Retrieved 2010-01-25 .
- ^ Marcucci, G. Thousand.; Polk, J. East. (2000). "NSTAR Xenon Ion Thruster on Deep Space i: Basis and flying tests (invited)". Review of Scientific Instruments. 71 (3): 1389–1400. Bibcode:2000RScI...71.1389M. doi:x.1063/1.1150468.
- ^ Sovey, James S.; Rawlin, Vincent K.; Patterson, Michael J. "A Synopsis of Ion Propulsion Development Projects in the United States: SERT I to Deep Space I" (PDF). NASA. Archived from the original (PDF) on June 29, 2009. Retrieved 2009-12-12 .
- ^ Marrese, C.; Polk, J.; Mueller, J.; Owens, A.; Tajmar, M.; Fink, R. & Spindt, C. (October 2001). In-FEEP Thruster Ion Beam Neutralization with Thermionic and Field Emission Cathodes. 27th International Electric Propulsion Conference. Pasadena, California. pp. 1–15. Archived from the original (PDF) on 2010-05-27. Retrieved 2010-01-25 .
- ^ "Infrared illumination compositions and articles containing the aforementioned". United States Patent 6230628. Freepatentsonline.com. Retrieved 2010-01-25 .
- ^ "LUU-xix Flare". Federation of American Scientists. 2000-04-23. Archived from the original on 2010-08-06. Retrieved 2009-12-12 .
- ^ Charrier, E.; Charsley, E. L.; Laye, P. G.; Markham, H. Yard.; Berger, B.; Griffiths, T. T. (2006). "Determination of the temperature and enthalpy of the solid–solid phase transition of caesium nitrate by differential scanning calorimetry". Thermochimica Acta. 445: 36–39. doi:10.1016/j.tca.2006.04.002.
- ^ Crickmore, Paul F. (2000). Lockheed SR-71: the secret missions exposed. Osprey. p. 47. ISBN978-1-84176-098-8.
- ^ National Research Quango (U.S.) (2001). Energy research at DOE—Was it worth it?. National Academy Press. pp. 190–194. doi:10.17226/10165. ISBN978-0-309-07448-3 . Retrieved 2010-09-26 .
- ^ Roskill Information Services (1984). Economics of Caesium and Rubidium (Reports on Metals & Minerals). London, Great britain: Roskill Data Services. p. 51. ISBN978-0-86214-250-six.
- ^ Chin, Cheng; Grimm, Rudolf; Julienne, Paul; Tiesinga, Eite (2010-04-29). "Feshbach resonances in ultracold gases". Reviews of Modern Physics. 82 (2): 1225–1286. arXiv:0812.1496. Bibcode:2010RvMP...82.1225C. doi:x.1103/RevModPhys.82.1225. S2CID 118340314.
- ^ "Cesium 239240". Sigma-Aldrich. 2021-09-17. Retrieved 2021-12-21 .
- ^ Information from The Radiochemical Transmission and Wilson, B. J. (1966) The Radiochemical Manual (2nd ed.).
- ^ Melnikov, P.; Zanoni, L. Z. (June 2010). "Clinical effects of cesium intake". Biological Trace Element Research. 135 (1–3): 1–9. doi:10.1007/s12011-009-8486-7. PMID 19655100. S2CID 19186683.
- ^ Pinsky, Carl; Bose, Ranjan; Taylor, J. R.; McKee, Jasper; Lapointe, Claude; Birchall, James (1981). "Cesium in mammals: Acute toxicity, organ changes and tissue accumulation". Periodical of Environmental Science and Health, Part A. 16 (5): 549–567. doi:10.1080/10934528109375003.
- ^ Johnson, Garland T.; Lewis, Trent R.; Wagner, D. Wagner (1975). "Astute toxicity of cesium and rubidium compounds". Toxicology and Applied Pharmacology. 32 (2): 239–245. doi:10.1016/0041-008X(75)90216-1. PMID 1154391.
- ^ Rundo, J. (1964). "A Survey of the Metabolism of Caesium in Human". British Journal of Radiology. 37 (434): 108–114. doi:x.1259/0007-1285-37-434-108. PMID 14120787.
- ^ Nishita, H.; Dixon, D.; Larson, M. H. (1962). "Accumulation of Cs and K and growth of bean plants in nutrient solution and soils". Found and Soil. 17 (two): 221–242. doi:10.1007/BF01376226. S2CID 10293954.
- ^ Avery, S. (1996). "Fate of caesium in the environment: Distribution between the abiotic and biotic components of aquatic and terrestrial ecosystems". Periodical of Environmental Radioactivity. 30 (two): 139–171. doi:10.1016/0265-931X(96)89276-9.
- ^ Salbu, Brit; Østby, Georg; Garmo, Torstein H.; Hove, Knut (1992). "Availability of caesium isotopes in vegetation estimated from incubation and extraction experiments". Annotator. 117 (3): 487–491. Bibcode:1992Ana...117..487S. doi:10.1039/AN9921700487. PMID 1580386.
- ^ Vinichuk, M. (2010). "Accumulation of potassium, rubidium and caesium (133Cs and 137Cs) in various fractions of soil and fungi in a Swedish woods". Science of the Full Environment. 408 (12): 2543–2548. Bibcode:2010ScTEn.408.2543V. doi:ten.1016/j.scitotenv.2010.02.024. PMID 20334900.
- ^ Smith, Jim T.; Beresford, Nicholas A. (2005). Chernobyl: Catastrophe and Consequences. Berlin: Springer. ISBN978-iii-540-23866-nine.
- ^ Eremeev, V. N.; Chudinovskikh, T. V.; Batrakov, G. F.; Ivanova, T. M. (1991). "Radioactive isotopes of caesium in the waters and nigh-water atmospheric layer of the Black Sea". Physical Oceanography. 2 (1): 57–64. doi:10.1007/BF02197418. S2CID 127482742.
- ^ Redman, H. C.; McClellan, R. O.; Jones, R. K.; Boecker, B. B.; Chiffelle, T. L.; Pickrell, J. A.; Rypka, E. W. (1972). "Toxicity of 137-CsCl in the Beagle. Early Biological Effects". Radiation Research. 50 (three): 629–648. Bibcode:1972RadR...l..629R. doi:10.2307/3573559. JSTOR 3573559. PMID 5030090.
- ^ "Chinese 'find' radioactive ball". BBC News. 2009-03-27. Retrieved 2010-01-25 .
- ^ Charbonneau, Louis (2003-03-12). "IAEA director warns of 'dirty flop' risk". The Washington Post. Reuters. p. A15. Archived from the original on 2008-12-05. Retrieved 2010-04-28 .
External links [edit]
- Caesium or Cesium at The Periodic Table of Videos (Academy of Nottingham)
- View the reaction of Caesium (most reactive metal in the periodic table) with Fluorine (about reactive non-metal) courtesy of The Purple Institution.
- Rogachev, Andrey Yu.; Miao, Mao-Sheng; Merino, Gabriel; Hoffmann, Roald (2015). "Molecular CsF5and CsF2+". Angewandte Chemie. 127 (28): 8393–8396. Bibcode:2015AngCh.127.8393R. doi:10.1002/ange.201500402.
Is Cesium A Transition Metal,
Source: https://en.wikipedia.org/wiki/Caesium
Posted by: shellenbargerjuplage.blogspot.com




0 Response to "Is Cesium A Transition Metal"
Post a Comment